
The physico-chemical certainties
- Ocean acidification has started and is fully detectable: since industrialisation began, the acidity of surface seawater has already increased of 30% [1], with a pH droped from about 8.2 to 8.1 in average.
- The rate of acidification is accelerating, directly following the acceleration in world CO2 emissions (+3.5% / year since 2000 compared to +0.9% / year in the 90’s, which is greater than projected for the worst-case scenario formulated a decade ago)
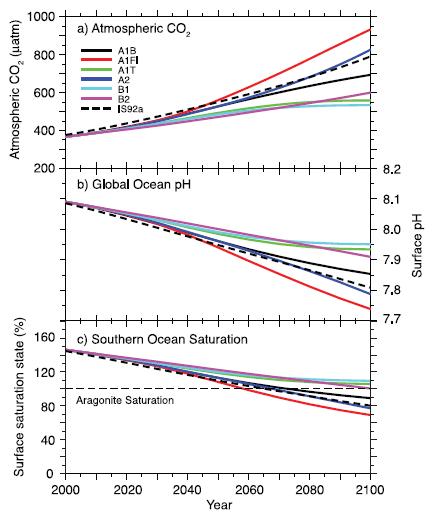 Changes in global average surface pH and saturation state with respect to aragonite in the Southern Ocean under various SRES scenarios. Time series of (a) atmospheric CO2for the six illustrative SRES scenarios, (b) projected
Changes in global average surface pH and saturation state with respect to aragonite in the Southern Ocean under various SRES scenarios. Time series of (a) atmospheric CO2for the six illustrative SRES scenarios, (b) projected
global average surface pH and (c) projected average saturation state in the Southern Ocean from the BERN2.5D EMIC (Plattner et al., 2001). The results for the SRES scenarios A1T and A2 are similar to those for the non-SRES scenarios S650 and IS92a, respectively. Modified from Orr et al. (2005). (IPCC, 2007)
- In addition to a decline in pH, the dissolution of CO2 in seawater also provokes a decrease in carbonate ions, the chemical compound that calcifying marine organisms such as corals, mollusks, crustaceans and urchins use to form their calcareous shells or skeletons. At the current pace of CO2 emissions, in only 10 years about 10% of Arctic surface waters will already become undersaturated with respect to aragonite, one of the natural forms of calcium carbonate (CaCO3). Such waters turn corrosive to CaCO3 and might cause shells and skeletons to dissolve. By 2100, all polar surface seawaters will become corrosive if emissions continue to increase as they currently do.
- Contrary to the greenhouse effect, which involves a lot of complex interactions, the physico-chemical reactions involved in acidification are straight-forward and can be predicted with full certainty for a given level of atmospheric CO2 concentration. Across the range of IPCC emission scenarios, surface ocean pH will decrease by 0.3 to 0.5 pH units relative to preindustrial conditions by 2100, which is a hundred times faster than any previous natural change that has occurred over the last many millions of years.
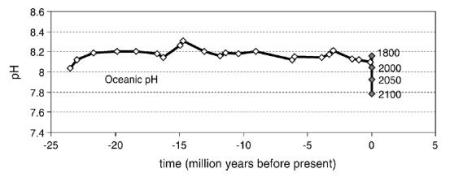 Past, present and future levels of average surface ocean pH. Future predictions based on IPCC scenarios (Blackford & Gilbert, 2007)
Past, present and future levels of average surface ocean pH. Future predictions based on IPCC scenarios (Blackford & Gilbert, 2007)
- Recovery will require thousands of years for the Earth system to reestablish ocean chemical conditions that even partially resemble those found today; and hundreds of thousands to millions of years will be needed for coral reefs to return, based on the past record of natural extinction events.
- The only way to stop ocean acidification is to stop increasing CO2 concentrations in the atmosphere.
[1] 30% more hydrogen ions (H+). The pH scale is logarithmic, therefore this increase in H+ is equivalent to a 0.1 unit decrease in pH.
[2] Predictions of greenhouse has emissions by human activities according to several socio-economic scenarios formulated by the Intergovernmental Panal on Climate Chage (IPCC).



